The novel coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has infected over 104 million lives and caused over 2.3 million deaths globally. Although predominantly considered a respiratory disease, one-fourth of the COVID-19 patients also present other symptoms, often related to the gastrointestinal or nervous systems.
Many other cells in the body besides lung alveolar epithelial cells express angiotensin-converting enzyme 2 (ACE2) receptors – the means through which the virus enters host cells. The heart, pancreas, GI tract, kidney, testis and other organs which express ACE2 receptors, and studies demonstrated that the virus infects the heart, vascular endothelial cells, kidney, liver and the pancreas.
In some cases, COVID-19 patients present acute pancreatitis – a condition where the pancreas-inflammation causes abdominal pain. Acute pancreatitis is also one of the most frequent gastrointestinal causes of hospitalization in the US. Currently, there is no knowledge on how SARS-CoV-2 infects the pancreas and what the impacts are.
To understand how SARS-CoV-2 infects the pancreas and investigate its effects, a team of researchers used the human iPSC (induced pluripotent stem cells)-derived pancreatic cultures and analyzed the post-infection cellular and molecular changes.
The interdisciplinary team from the Cedars-Sinai Medical Center, and the University of California, Los Angeles in the U.S. report their observations on the preprint server, medRxiv*. They demonstrate for the first time that SARS-CoV-2 can directly infect the human iPSC-derived pancreatic cells, with supporting evidence of the presence of the virus in post-mortem pancreatic tissue of confirmed COVID-19 human cases.
They found that SARS-CoV-2 infection of infecting iPSC-derived pancreatic cells increased a key inflammatory cytokine, CXCL12. It is known to be involved in pancreas dysfunction. They observed that the SARS-CoV-2 hijacked the ribosomal machinery in the pancreatic cells and also increased the expression of some pancreatic ductal stress response genes. Prominently, the genes CXCL12, NFKB1, and STAT3 showed significant upregulation as compared to the control.
The researchers report that the transcriptional analysis of SARS-CoV-2 infected iPSC-derived pancreatic cultures demonstrated active viral replication and pancreas-specific COVID-19 associated disease signatures. The SRP-protein targeting processes were upregulated, indicating that host cell machinery was being repurposed for viral replication.
For this study, established cell lines do not represent the human pancreas pathophysiology. Because the primary human pancreatic cells are largely inaccessible, the researchers developed novel methods to generate pancreatic progenitors from human induced pluripotent stem cells (iPSCs), which can be differentiated into endocrine (islet β-cells) and exocrine (acinar and ductal) cells.
The researchers generated the highly stable iPSCs from healthy volunteers at the iPSC Core at Cedars-Sinai Medical Center from the peripheral blood mononuclear cells (PMBCs) utilizing non-integrating oriP/EBNA1-based episomal plasmid vectors. These human iPSCs were differentiated into pancreatic adherent (2D) and organoid (3D) cultures.
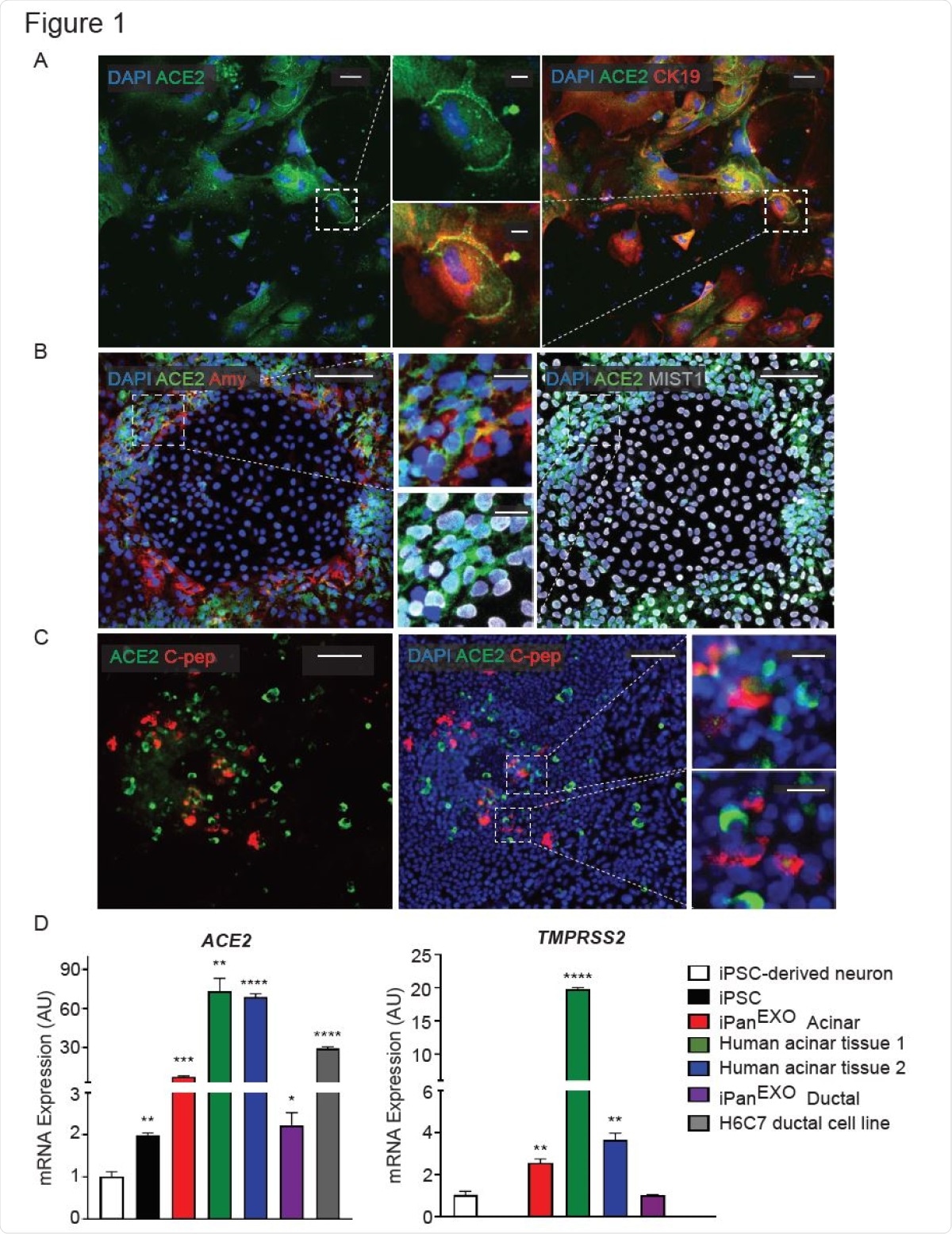
These cultures contain cells that are representative of human endocrine pancreas expressing endocrine NKX6.1 and C-peptide, exocrine acinar Amylase and Chymotrypsin (CTRC) and exocrine ductal Cytokeratin19 (CK19) and SOX9 cells, the researchers write.
From RNA-sequencing studies, they established that the pancreas, specifically the exocrine compartment (acinar and ductal cells), has high expression of ACE2. Gender and age present no difference in the expression of the ACE2 receptors. The researchers demonstrated that the iPSC-derived pancreatic cells used in this study exhibit ACE2 and TMPRSS2 expression. Both the receptors are present in the pancreas, especially in the exocrine portion.
In the context of the endocrine pancreas, it is well-established that patients with type I and type II diabetes have a higher risk for COVID-19 associated mortality. Diabetic COVID-19 patients, with associated comorbidities such as cardiovascular diseases and renal impairment, succumb to the severity of the infection. It is thus important to know the mechanism of the SARS-CoV-2 infection on human endocrine and exocrine pancreas.
The researchers show that the pancreatic cells including the endocrine and the exocrine cell types (acinar, and ductal) are susceptible to SARS-CoV-2 infection, resulting in morphological perturbations as well as impaired expression of key markers.
The researchers have demonstrated for the first time that SARS-CoV-2 can directly infect human iPSC-derived pancreatic cells. They support this with evidence of the presence of the virus in post-mortem pancreatic tissue of confirmed COVID-19 human cases. Corresponding with the infection, the study shows important inflammatory signatures.
The iPSC-based model described here provides a valuable novel platform for understanding the pancreas-specific cellular responses to SARS-CoV-2 as well as for antiviral drug development against SARS-CoV-2. ”
This study presents a novel model of iPSC-derived pancreatic cultures as a new avenue for understanding the SARS-CoV-2 infection. It potentially establishes an excellent platform for endocrine and exocrine pancreas-specific antiviral drug screening, the researchers write.
*Important Notice
medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
https://news.google.com/__i/rss/rd/articles/CBMiV2h0dHBzOi8vd3d3Lm5ld3MtbWVkaWNhbC5uZXQvbmV3cy8yMDIxMDIwNS9Ib3ctZG9lcy1TQVJTLUNvVi0yLWluZmVjdC10aGUtcGFuY3JlYXMuYXNweNIBW2h0dHBzOi8vd3d3Lm5ld3MtbWVkaWNhbC5uZXQvYW1wL25ld3MvMjAyMTAyMDUvSG93LWRvZXMtU0FSUy1Db1YtMi1pbmZlY3QtdGhlLXBhbmNyZWFzLmFzcHg?oc=5
2021-02-05 12:33:00Z
52781353865918
Bagikan Berita Ini














0 Response to "How does SARS-CoV-2 infect the pancreas? - News-Medical.Net"
Post a Comment