 Thought LeadersProfessor Deborah GoberdhanAssociate Professor of Cell SignallingUniversity of Oxford
Thought LeadersProfessor Deborah GoberdhanAssociate Professor of Cell SignallingUniversity of OxfordProfessor Deborah Goberdhan speaks to News-Medical about her team's latest research into cancer cells, and the mechanisms they use to communicate and adapt to stress.
What provoked your research into cancer cells and their communication?
I was unexpectedly provoked into cancer research during my doctoral studies, through the discovery of some remarkable tumor-like overgrowths in flies carrying an unidentified mutation. The culprit turned out to be the fly equivalent of PTEN, which had just been identified as a major human tumor suppressor gene.
We showed that PTEN was lost in the enlarged cells contributing to these overgrowths and had a central role in growth factor signaling. Our fly studies subsequently highlighted an amino acid-sensing mechanism as a means of selectively blocking cancer growth.
One of the early goals of my new research group at Oxford was to determine how this mechanism worked in human cells. These studies showed that cells sensed their environment by engulfing extracellular fluid through a process called endocytosis and then detecting nutrients, such as amino acids, from the surface of the resulting fluid-filled structures, termed endosomes and lysosomes.
Meanwhile, I became interested in communication between cells through the serendipitous identification of exosomes in a fly gland.
Image Credit: Javier Regueiro/Shutterstock.com
What are exosomes and what is their role within cancer?
Exosomes are a type of small membrane-surrounded, secreted vesicle. Until recently, they were thought to be made inside a particular class of endosomes, the late endosomes. Individual exosomes transfer a diverse mixture of molecules, including proteins, RNA, and DNA to target cells, and can induce behavioral changes in those cells.
A plethora of studies have implicated exosomes in cancer adaptation and progression. For example, primary tumor cell exosomes can reprogram normal cells to support both local tumor growth and the distant growth of metastasizing tumor cells. Exosomes can also suppress the immune system, causing patients to respond less well to immunotherapies.
Developing a better understanding of how exosomes are made and thus, how they can be blocked may provide a means of stopping cancer cells from fighting against therapies, developing metastases, and impacting on the immune system.
Can you describe how you carried out your research that led to the discovery of Rab11a-exosomes role in cancer?
Our discovery of exosomes in flies led to the development of a powerful approach to studying how they are made and the identification of Rab11a-exosomes as a new type of human exosome. This fly model has provided a simple means of investigating the genetic control of exosome formation, facilitated by the unusually large endosomal compartments of some of its exosome-producing cells.
We have been able to see exosomes being made, for the first time, in dissected living tissue using high-resolution microscopy. This has enabled us to observe the fly equivalent of Rab11a-exosomes and show that they are a distinct exosome subtype located within Rab11-recycling endosomes, a new exosome-generating compartment.
Lack of external resources, such as glutamine, which affects cancer cell homeostasis, leads to an increase in the production of human Rab11a-exosomes. This commonly happens in the poorly vascularized core regions within a growing tumor. The switch to Rab11a-exosome production also happens in response to drugs that target the microenvironmental sensor mTORC1 and reduced levels of the PAT4 amino acid sensor, which we have shown controls mTORC1 activity.
Functional impact of Rab11a-exosome production was assessed by comparing the effect of adding Rab11a-exosome-enriched and non-enriched vesicle preparations to naive cells and determining the effect on, for example, cancer and blood vessel growth in cell culture and human tumors formed in mice, so-called xenograft models.
This work has also highlighted molecules such as AREG, a ligand for the growth factor receptor EGFR, as a potential causative cargo that could be targeted therapeutically. I head a Cancer Research UK Programme, which has supported this work. It has evolved out of a close and very enjoyable collaboration between three groups: the first led by Professor Adrian Harris, a clinical oncologist, the second by Professor Clive Wilson, a fly geneticist, and my own, which bridges the gap between the two.
The heterogeneity of exosomes has been apparent for some time, but up until now, it has not been appreciated that endosomal origin is a key contributory factor and that changing the environmental conditions of a cell can lead to a change in exosome subtype and the intercellular messages they carry.
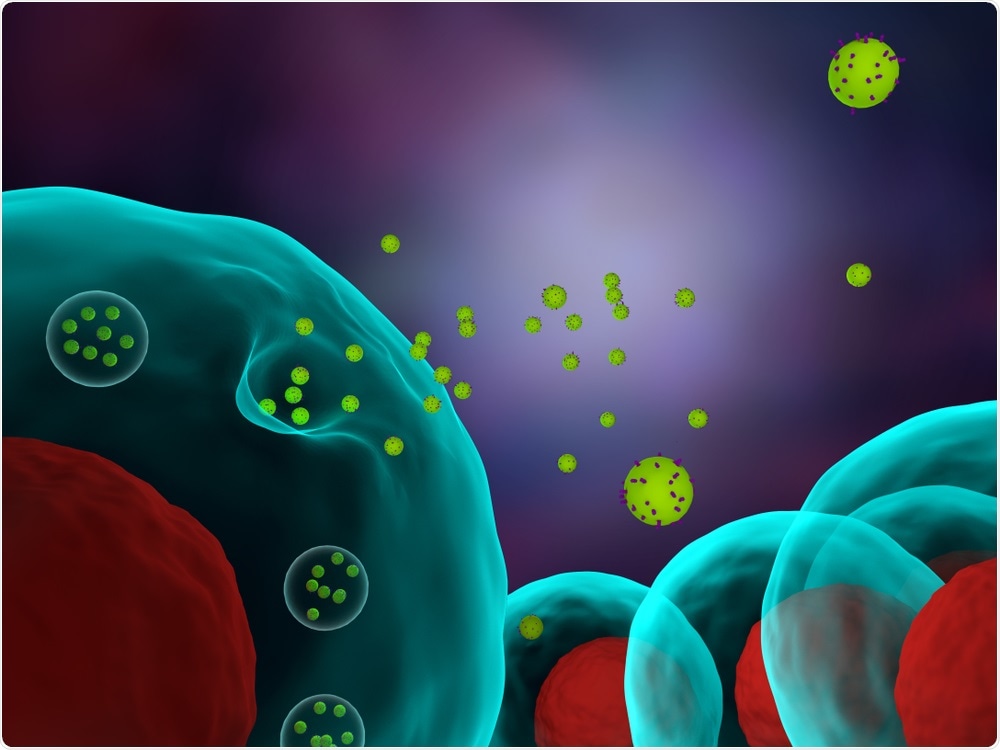
Image Credit: Meletios Verras/Shutterstock.com
How do Rab11a exosomes lead to the unresponsiveness of some cancer therapies and resistance in others?
The generation and secretion of Rab11a-exosomes are suppressed by high levels of nutrients, especially glutamine, and also by growth factor signaling, which is frequently elevated because of mutations in cancer cells.
When tumor growth outstrips the availability of nutrients or we block growth factor signaling in many of the most common cancer therapies, Rab11a-exosomes are preferentially secreted. They are loaded with molecules that can provide an additional growth-promoting boost to other cancer cells in a nutrient-deprived area of the tumor, and that can also both stimulate blood vessels to grow to increase the nutrient supply and suppress the immune system.
We hypothesize that this leads to the selection of populations of cancer cells that are more prolific and less responsive to therapy. These more aggressive cancer cells increase in number and drive tumor progression.
Do you believe that by identifying the role of this exosome, we can help to improve cancer therapies and their outcomes?
Yes. We now have many drugs that can block tumor growth, but very often the tumor develops resistance mechanisms and an important focus in designing combinatorial therapies is to target those confounding effects at the same time.
Working out ways of blocking Rab11a-exosome secretion may provide one answer or we may be able to identify other proteins on Rab11a-exosomes and use antibodies to block the pro-tumorigenic functions of these vesicles.
While our ability to therapeutically target Rab11a-exosomes may take some years to develop, understanding how Rab11a-exosomes work in resistance mechanisms may help us to customize current treatments to specific patients and types of cancers with improved patient outcome.
What other potential impacts can this exosome have on early detection of cancer and subsequent cancer survival?
A major stumbling block in analyzing exosome and other extracellular vesicles is their heterogeneous nature. The identification of Rab11a-exosomes has highlighted a specific pro-tumorigenic exosome subtype, with a different protein and RNA signature to other exosomes.
As our knowledge of the cancer cell Rab11a-exosome specific signature increases, the opportunities to detect these exosomes in body fluids will increase, leading to earlier detection with improved patient benefit and survival. With the potential to enhance early disease detection, there are many groups around the world trying to develop routine blood tests for exosomes associated with cancer and other disorders, such as Alzheimer’s disease.
The identification of Rab11a-exosomes is likely to impact on these studies because it highlights a specific exosome subtype that is relevant to disease. If we can co-detect multiple markers on a single type of exosome, this will increase the specificity of these blood tests. The link between Rab11a-exosomes and tumor resistance also means that their detection may enable us to determine which patients will respond best to specific treatments.

Image Credit: Roman Zaiets/Shutterstock.com
What further research and information is needed before we can look to utilize this research for cancer therapies?
In the long term, we need to gain better insights into how Rab11a-exosomes are made. The combination of our fly exosome formation model and human cancer cell line approaches has proved an effective means of doing this with several different Rab11a-exosome formation mechanisms already under test.
These mechanistic clues will then be validated in mice. In the shorter term, we need to characterize Rab11a-exosomes in more detail, so that we can use them as multifactorial biomarkers. This will involve developing improved separation methods, such as specific capture with antibodies against surface Rab11a-exosome markers, and a routine method to detect the molecules inside them.
One approach that several groups are developing, including one of our collaborators, is a technique called microfluidics. The goal of this method is the development of a simple detector that requires less than a milliliter of patient blood, similar to the ones used to measure blood insulin.
What are the next steps in your research into exosomes and cancer?
We are continuing to identify selective ways of blocking Rab11a- and other pro-tumorigenic exosome subtypes. This work has highlighted groups of molecules that are specifically involved in generating Rab11a-vesicles, which could lead to new treatments for blocking these vesicles.
We are also interested in defining the cargos on different exosome subtypes, which may provide a means of detecting cancer earlier with improved benefits for patients. This involves using approaches such as proteomics and transcriptomics to screen for markers that are consistently present on Rab11a-exosomes from cancer cells, but not more normal cells. We are also characterizing other exosome subtypes in flies, before further characterization in human cancer cells.
There have already been several spin-offs to our work. Through a collaboration with the group of Professor Matthew Wood in Oxford, we have found that a molecule that appears to bind at high levels to the outside of Rab11a- and other exosomes (GAPDH) can be repurposed as a carrier for biomolecules, such as siRNAs, which can be used in genetic therapies.
This technology seems to apply to exosomes from many sources, with an equivalent system existing in flies. By repurposing patient exosomes and thereby avoiding immunogenicity issues, this opens up the possibility to deliver therapies of choice to specific target cells in patients.
Where can readers find more information?
The full paper is available to read in The EMBO Journal The EMBO Journal has also published a review about the paper, written by two leaders in the extracellular vesicle field, in the same issue. This can be read via this link: Extracellular vesicles: eat glutamine and spit acidic bubbles.
More recent work highlighting a new mechanism to block Rab11a-exosomes can be found here: https://www.biorxiv.org/content/10.1101/2020.06.18.158725v1
While other collaborative work suggests a role for GAPDH in enhancing the therapeutic potential of extracellular vesicles in drug delivery to the brain: BioRxiv, https://doi.org/10.1101/2020.01.09.899880.
More on Prof Goberdhan’s research can be found here https://www.dpag.ox.ac.uk/team/deborah-goberdhan
About Professor Deborah Goberdhan
Deborah Goberdhan is Associate Professor of Cell Signalling in the Department of Physiology, Anatomy, and Genetics at the University of Oxford, where she heads a Cancer Research UK Programme, focusing on exosome and other extracellular vesicle (EV) signaling, its regulation by microenvironmental stress and its roles in cancer cell adaptation.
She studied Chemistry at Oxford, then trained at Harvard, MIT and the University of Kent, before setting up her own lab in Oxford. Her group has made several discoveries in basic cancer biology relating to growth factors and other cell signaling, stress responses, and nutrient sensing.
Her team currently uses a collaboratively developed in vivo fly model of exosome biogenesis and complementary analysis in human cancer cell lines to investigate fundamental aspects of exosome and EV biology with a view to applying these findings clinically, as discussed above. This approach is providing further insights into subtype-specific regulators of exosome biogenesis, the design of new bio-delivery tools, and into novel extracellular, multiplex signaling complexes they have collaboratively identified, termed microcarriers.
Deborah is actively involved in bringing together EV and exosome work in Oxford through an EV focus group and has recently been elected on to the Executive Board of the International Society for Extracellular Vesicles (ISEV), the leading professional society in the EV field.
https://news.google.com/__i/rss/rd/articles/CBMiYGh0dHBzOi8vd3d3Lm5ld3MtbWVkaWNhbC5uZXQvbmV3cy8yMDIwMDgyNi9FeG9zb21lcy1UaGUtY2FuY2VyLWNlbGwtY29tbXVuaWNhdGlvbi1tZWNoYW5pc20uYXNweNIBZGh0dHBzOi8vd3d3Lm5ld3MtbWVkaWNhbC5uZXQvYW1wL25ld3MvMjAyMDA4MjYvRXhvc29tZXMtVGhlLWNhbmNlci1jZWxsLWNvbW11bmljYXRpb24tbWVjaGFuaXNtLmFzcHg?oc=5
2020-08-26 12:52:00Z
CBMiYGh0dHBzOi8vd3d3Lm5ld3MtbWVkaWNhbC5uZXQvbmV3cy8yMDIwMDgyNi9FeG9zb21lcy1UaGUtY2FuY2VyLWNlbGwtY29tbXVuaWNhdGlvbi1tZWNoYW5pc20uYXNweNIBZGh0dHBzOi8vd3d3Lm5ld3MtbWVkaWNhbC5uZXQvYW1wL25ld3MvMjAyMDA4MjYvRXhvc29tZXMtVGhlLWNhbmNlci1jZWxsLWNvbW11bmljYXRpb24tbWVjaGFuaXNtLmFzcHg
Bagikan Berita Ini















0 Response to "Exosomes: The cancer cell communication mechanism - News-Medical.net"
Post a Comment