Study setting and study participants
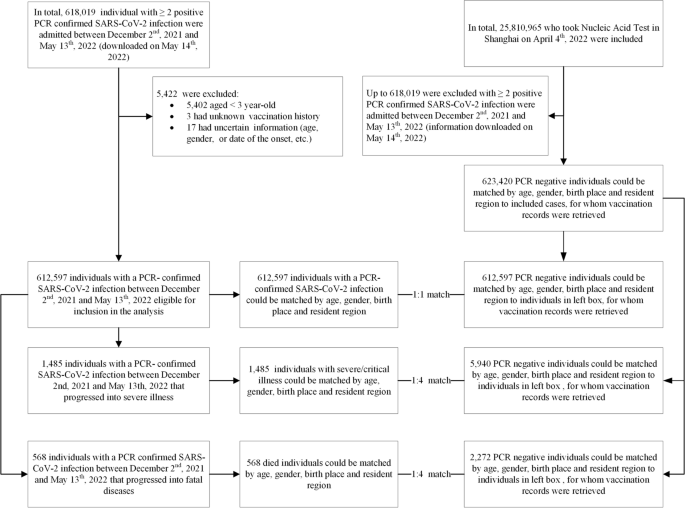
The study was conducted among people living in Shanghai, a provincial-level municipality in China with a resident population of more than 25 million people. Everyone living in Shanghai—citizens, foreigners, and immigrants—underwent several rounds of SARS-CoV-2 real-time polymerase chain reaction (RT-PCR) testing between 2 December 2021 and 13 May 2022. Complete demographic (age, gender, birthplace, and residential district), vaccination, and RT-PCR testing data were available to public health officials and study investigators. Individuals with negative RT-PCR SARS-CoV-2 tests who had tested positive before 2 December 2021 were excluded. Children less than 3 years of age were not vaccine-eligible and were excluded.
Controls were selected from consecutively test-negative individuals at the same time as cases were diagnosed. The size of the potential control group was 25.18 million people receiving several rounds of city-wide nucleic acid amplification testing (NAAT), and RT-PCR testing was universal regardless of COVID-19-associated symptoms.
Study design
We used a matched case-control design for data extraction. Cases were individuals with documented SARS-CoV-2 infection, severe or critical COVID-19, or COVID-19-related death. Infection-only cases were matched 1:1 with controls on year-of-age, gender, birthplace (Shanghai/other places), date of testing, and residential district; severe or critical COVID-19 cases and COVID-19-related death cases were matched 1:4 on year-of-age, gender, birthplace, date of illness onset, and residential district (Fig. 1). We matched each case to unique controls without replacement. When there is more than one case-control pair available, we selected the first (for infection-only cases) or first four (for severe/critical and fatal cases) eligible individuals. We constructed two subsets to analyze separate VEs of inactivated vaccines (subset 1) and Ad5-vectored vaccine (subset 2) against each of the three outcomes (Additional file 1: Figs. S1 and S2).
Infections and outcomes
PCR testing for SARS-CoV-2 is available in public hospitals and private laboratories throughout China. Since 10 March 2022, Shanghai conducted several rounds of SARS-CoV-2 tests, each involving more than 25 million people. The severity of disease for all SARS-CoV-2 infections was assessed in any of 48 COVID-19-designated hospitals in accordance with the Diagnosis and Treatment Protocol for COVID-19 (trial version 9) [15]. We included three outcomes in our study: documented SARS-CoV-2 infection, severe/critical COVID-19, and COVID-19-related death. Documented SARS-CoV-2 infection was confirmed by a positive RT-PCR test. For adult cases, severe illness must meet any of the following criteria: (a) respiratory distress (respiration rate [RR] ≥ 30 breaths per minute (BPM)), (b) oxygen saturation ≤ 93% at rest, and (c) arterial partial pressure of oxygen/fraction of inspired oxygen ≤ 300 mmHg. Additionally, cases with chest imaging that shows obvious lesion progression within 24–48 h > 50% shall be managed as severe illness. For child cases, severe illness must meet any of the following criteria: (a) persistent high fever over 3 days; (b) tachypnea (RR ≥ 60 BPM for infants aged below 2 months; RR ≥ 50 BPM for infants aged 2–12 months; RR ≥ 40 BPM for children aged 1–5 years, and RR ≥ 30 BPM for children > 5 years), independent of fever and crying; (c) oxygen saturation ≤ 93% on finger pulse oximeter taken at rest; (d) labored breathing (moaning, nasal fluttering, and infrasternal, supraclavicular, and intercostal retraction), cyanosis, and intermittent apnea; (e) lethargy and convulsion; and (f) difficulty feeding and signs of dehydration. Critical illness must meet any of the following criteria: (a) respiratory failure and requiring mechanical ventilation, (b) shock, and (c) other organ failures that require intensive care unit (ICU) care. COVID-19-related death is assessed by medical institutions.
Data on all confirmed and asymptomatic cases between 2 December 2021 and 13 May 2022 were obtained from the National Notifiable Diseases Registry System (NNDRS).
Vaccination data
In this study, inactivated vaccine included Sinovac-CoronaVac, Sinopharm/BIBP COVID-19 vaccine, and Sinopharm/WIBP COVID-19 vaccine; Ad5-vectored vaccine referred to Cansino Ad5-nCoV-S COVID-19 vaccine; and recombined protein vaccine referred to recombinant SARS-CoV-2 vaccine (CHO cell), Anhui Zhifei Longcom Biopharmaceutical Institute of Microbiology.
The Shanghai Group Immunization System captures all vaccine administrations and is updated daily. This immunization information system is the repository of nearly all vaccinees’ records and includes the name, national identification number, vaccine type, vaccination date, vaccination dose, vaccination site, and vaccine manufacturer. This system is linked to the National Immunization Program Information System, which adds documented national identification-matching COVID-19 vaccinations received outside of Shanghai. Immunization histories were verified manually with records for subjects 3 years and older who had an unknown national identification number. NAAT data were linked to individual vaccination records using national identification numbers and names. Vaccination data were extracted and matched to cases and controls on 13 May 2022. Additional file 1: Fig. S3 shows the process of vaccination history retrieval.
Vaccination status was categorized into four levels in accordance with the national technical recommendations for COVID-19 vaccination: (1) unvaccinated—either no history of COVID-19 vaccination before the last SARS-CoV-2 exposure date; (2) partial vaccination—either one dose of inactivated vaccine, or two doses of inactivated vaccine but receiving the second dose within 14 days before the last SARS-CoV-2 exposure date, or two doses of recombinant protein vaccine (three doses are recommended for primary vaccination), or one dose of adenovirus vector vaccine or three doses of recombinant protein vaccine but with the most recent dose within 14 days before the last SARS-CoV-2 exposure date; (3) full primary vaccination—either two doses of inactivated vaccine, one dose of adenovirus vector vaccine, three doses of recombinant protein (CHO cell) vaccine with the most recent doses 14 days or more before the last SARS-CoV-2 exposure date and with no booster dose; or two doses of inactivated vaccine with one booster dose of inactivated vaccine, adenovirus vector vaccine, or recombinant protein (CHO cell) vaccine within 7 days before the last SARS-CoV-2 exposure date; or two doses of adenovirus vector vaccine within 7 days before the last exposure date; or (4) booster vaccination—either two doses of Ad5-vectored vaccine with the second dose 7 days or more before the last exposure date or two doses of inactivated vaccine and one booster dose of inactivated vaccine, adenovirus vector vaccine, or recombinant protein vaccine 7 days or more before the last exposure date.
Vaccination intervals were stratified by dose and interval (in weeks) post-vaccination at < 2, 3–12, 13–24, 25–36, and 37+ weeks after the first dose; < 2, 3–12, 13–24, 25–36, and 37+ weeks after the second dose; and < 2, 3–12, 13–24, and 25+ weeks after the third dose. Intervals (in days) were defined as the number of days between the onset date of infection and the date of the last vaccination minus 2 days, which was then converted into weeks. Detailed variable definitions are in Additional file 1: Table S1. Additionally, we performed a sensitivity analysis in which intervals (in days) were defined as the number of days between the testing positive date and the date of the last vaccination minus 7 days.
Statistical analysis
Non-normally distributed continuous variables were expressed as medians (interquartile ranges (IQR)), and categorical variables were expressed as counts and proportions. Conditional logistic regression was used to estimate the odds ratio (OR) of vaccination among cases and controls, with documented SARS-CoV-2 infection, severe or critical COVID-19, and COVID-19-related death as dependent variables. Vaccination status was the independent variable, and VE was defined as 1 minus the matched OR. Vaccination status was defined using the date of onset and date of last vaccination, as above. Analyses were stratified by age category, gender, and vaccine type (inactivated vaccine, adenovirus vector vaccine, and recombinant protein vaccine). Matching was conducted with the use of JAVA, and analyses were performed with the use of RStudio 2022.02.3+492.
https://news.google.com/__i/rss/rd/articles/CBMiSWh0dHBzOi8vYm1jbWVkaWNpbmUuYmlvbWVkY2VudHJhbC5jb20vYXJ0aWNsZXMvMTAuMTE4Ni9zMTI5MTYtMDIyLTAyNjA2LTjSAQA?oc=5
2022-10-20 13:06:29Z
1619062186
Bagikan Berita Ini














0 Response to "Effectiveness of inactivated and Ad5-nCoV COVID-19 vaccines against SARS-CoV-2 Omicron BA. 2 variant infection, severe illness, and death - BMC Medicine - BMC Medicine"
Post a Comment