Clinical record
A 75‐year‐old man presented with three days of lethargy and diarrhoea. He had a background of previous symptomatic acute digoxin toxicity requiring digoxin‐specific antibody fragments treatment three years previously, as well as metastatic prostate cancer, atrial fibrillation, ischaemic heart disease, mild cognitive impairment, and type 2 diabetes. Admission digoxin level was 14.0 μg/L (ARCHITECT i2000SR chemiluminescent micro‐particle immunoassay, Abbott Laboratories; therapeutic range, 0.5–1.1 μg/L; toxic, > 3.0 μg/L; lethal, > 12 μg/L). He was admitted for cardiac monitoring and consideration of digoxin‐specific antibody fragments administration. However, unlike his previous presentation with acute digitalis toxicity, his electrocardiogram was normal and there was no bradycardia, hypotension, nausea or renal impairment. Repeat digoxin levels six and 12 hours after admission yielded levels of 17 μg/L and 12 μg/L respectively.
The patient was unable to list his current medications on admission. A medication review conducted the next day confirmed that digoxin had been ceased following the previous toxic episode three years prior, no scripts for digoxin had been filled and the patient's spouse was not taking digoxin. A family member confirmed there were no digoxin containers in the house. The patient's other medications included enzalutamide for prostate cancer, oral hypoglycaemic agents, and heart failure therapy.
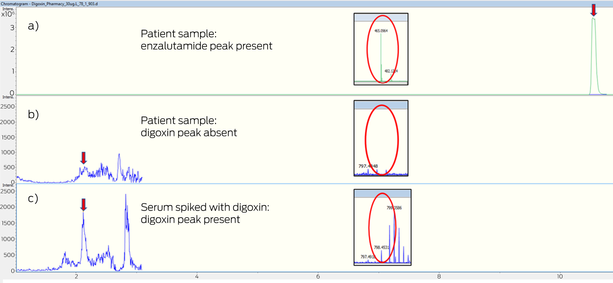
All three digoxin samples were analysed on two alternate digoxin assays. The Roche electrochemiluminescence immunoassay (Cobas e601, Roche Diagnostics) yielded a level of < 0.4 μg/L, and quadrupole time‐of‐flight mass spectrometry (QTOF‐MS; Impact II, Bruker) analysis confirmed the absence of digoxin and presence of enzalutamide, confirming the Abbott immunoassay falsely identified digoxin in the presence of enzalutamide (Box). The previous admission with digoxin toxicity due to acute renal injury pre‐dated enzalutamide commencement. The lethargy was related to enzalutamide; faecal microscopy was unremarkable, and diarrhoea subsequently improved with conservative management.
Discussion
Digoxin use has declined since the 1990s but toxicity remains an issue, with over 139 patients admitted to Australian hospitals with cardiac glycoside toxicity in 2011–12.1 Manifestations of toxicity include gastrointestinal symptoms, lethargy, confusion and cardiac dysrhythmias.1
The use of digoxin‐specific antibody fragments is indicated for the treatment of life‐threatening digoxin toxicity. The guidelines for the indication of digoxin‐specific antibody fragments vary, and include life‐threatening arrhythmia, cardiac arrest, hyperkalaemia above 5.0 mmol/L, along with moderate to severe gastrointestinal symptoms and serum digoxin concentration greater than 12 μg/L.1 The variations in guidelines are based on cost–benefit analysis, with each digoxin‐specific antibody fragments ampoule costing $1000 and each patient may require multiple ampoules.1 Digoxin‐specific antibody fragments are also associated with adverse effects, including electrolyte disturbances and, rarely, decompensated heart failure and atrial fibrillation with rapid ventricular response.1
Enzalutamide is an increasingly used competitive androgen receptor antagonist for use in metastatic castrate‐resistant prostate cancer and has been shown to prolong survival compared with placebo.2 It has a more desirable side‐effect profile than standard androgen deprivation therapy and is becoming increasingly favoured with the ageing population.2
While prostate cancer is the most prevalent cancer in older men, and the incidence of heart failure and renal impairment increases with age,2 the combined use of these two medications has the potential for misdiagnosis and mismanagement of digitalis toxicity. Digoxin is routinely analysed via automated immunoassays, and digoxin‐like immunoreactive substances are known to cause interference depending on the reagent's antibody specificity.3 Mass spectrometry detects digoxin via mass to charge ratio and overcomes the non‐specificity of antibody binding inherent in immunoassays. A clinical case reported a patient taking enzalutamide and digoxin whose mass spectrometry assay two weeks after digoxin had been ceased revealed a true digoxin level of 0.3 μg/L, with a falsely toxic Abbott digoxin immunoassay result of 8.8 μg/L, and a falsely high Beckman Coulter immunoassay result of 1.6 μg/L.4 The Roche, Siemens and Thermo Fisher digoxin assays were unaffected. We were able to simultaneously confirm the presence of enzalutamide and the absence of digoxin using the QTOF‐MS analyser, permitting a search for an alternate diagnosis and saving the patient from expensive and potentially harmful exposure to digoxin‐specific antibody fragments.
Clinicians should be aware of this new technology, with the potential for rapid simultaneous targeted screening for over 1000 therapeutic drugs and metabolites,5 and its implications for recognising medication non‐compliance across a multitude of clinical scenarios, in addition to intentional, surreptitious and malicious intake of non‐prescribed drugs.6 The rapid confirmation or exclusion of therapeutic or recreational drugs with high specificity can assist with acute presentation with uncertain drug history. In addition, clinicians should be aware of the potential for falsely elevated digoxin levels on some immunoassays in patients taking enzalutamide, which is an increasingly prescribed drug for castrate‐resistant prostate cancer in Australia.
Lessons from practice
- Enzalutamide can result in false positive digoxin levels on specific digoxin assays, leading to misdiagnosis and potential mismanagement.
- Understanding the laboratory assays available can help delineate between false toxic and true toxic results.
- The quadrupole time‐of‐flight mass spectrometry analysis (QTOF‐MS) is a new technology that has the potential for rapid simultaneous targeted screening for over 1000 therapeutic drugs and metabolites, which can confirm the presence of enzalutamide and absence of digoxin.
- The QTOF‐MS technology can assist with navigating uncertainty in medication non‐compliance, intentional, surreptitious or malicious intake of prescribed and non‐prescribed drugs.
Patient consent
The patient gave written consent for publication.
Box – Mass spectroscopy chromatogram: (a) patient plasma with enzalutamide peak with extracted ion chromatogram adduct (465 m/z); (b) in the same patient, plasma sample with undetectable digoxin peak, and undetectable digoxin ammoniated adduct at 798 m/z; and (c) plasma control sample spiked with digoxin (30 μg/L) with detected digoxin peak and extracted ion chromatogram digoxin ammoniated adduct (798 m/z)
https://news.google.com/rss/articles/CBMiYmh0dHBzOi8vd3d3Lm1qYS5jb20uYXUvam91cm5hbC8yMDI0LzIyMC85L21lYXN1cmVtZW50LWRpZ294aW4tbGV2ZWxzLWZhbHNlbHktYWZmZWN0ZWQtZW56YWx1dGFtaWRl0gEA?oc=5
2024-05-05 16:45:21Z
CBMiYmh0dHBzOi8vd3d3Lm1qYS5jb20uYXUvam91cm5hbC8yMDI0LzIyMC85L21lYXN1cmVtZW50LWRpZ294aW4tbGV2ZWxzLWZhbHNlbHktYWZmZWN0ZWQtZW56YWx1dGFtaWRl0gEA
Bagikan Berita Ini














0 Response to "Measurement of digoxin levels falsely affected by enzalutamide - The Medical Journal of Australia"
Post a Comment