The COVID-19 pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has affected over 30 million individuals worldwide. Originating in Wuhan (China) at the end of 2019, the virus and has claimed over 959,000 lives to date. While most patients present with fever, cough, and fatigue, some progress to acute respiratory distress syndrome or other critical and often fatal conditions.
The severity of COVID-19 disease is linked to age and comorbidities, including coronary heart disease, diabetes, and hypertension. Although the mechanism of disease is still not entirely understood, more and more studies show strong evidence for a significant role in the immune system. The pathophysiology of COVID-19 relies on the activation of the immune system and lymphopenia, which is a prognosis marker. Some evidence suggests that myeloid cells may be involved in COVID-19 pathophysiology, either directly as the virus target or indirectly.
A team of researchers from Aix-Marseille Univ, IHU-Méditerranée Infection, Institut Paoli Calmettes and Laboratoire d’Immunologie, Marseille, France, conducted experiments to determine if SARS-CoV-2 can affect the myeloid compartment or infect monocytes and macrophages.
Monocytes are innate hematopoietic cells that help maintain vascular homeostasis and drive early responses to microorganisms during acute infections. There are three human monocyte subsets based on the expression of CD14 / CD16 surface antigens. They are classical CD14+CD16- monocytes, intermediate CD14+CD16+ monocytes, and non-classical CD14-CD16+ monocytes.
About the study
In the study, published in the preprint server bioRxiv*, 76 SARS-CoV-2 patients were confirmed positive through reverse transcriptase-polymerase chain reaction (RT-PCR). Within 48 hours of diagnosis, the patients underwent clinical and blood tests. Peripheral blood mononuclear cells (PBMCs) were isolated from the blood of these patients and from healthy blood donors who acted as controls. The cells were infected with 50 µl of SARS-CoV-2 virus suspension.
Viral RNA was extracted from monocytes and macrocytes, and the levels of IL-10, tumor necrosis factor (TNF)-α, IL380 1β, interferon (IFN)-β, and transforming growth factor (TGF)-β1 were quantified using specific immunoassay kits.
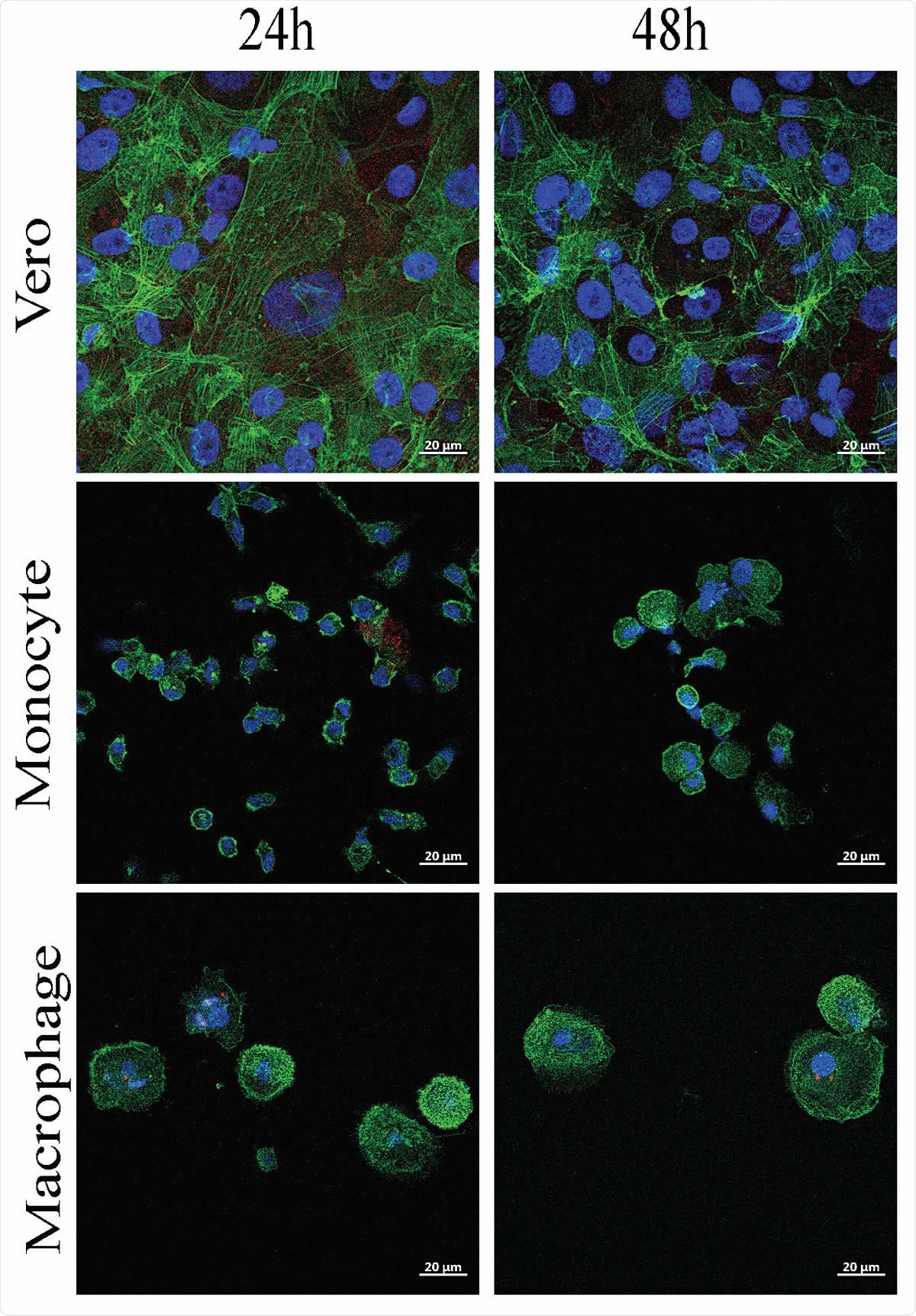
Monocytes and macrophages are targets of SARS-CoV-2
The study showed that SARS-CoV-2 could infect monocytes and macrophages and stimulate cytokine release without any cytopathic effect. The team found that SARS-CoV-2 infection was linked to the secretion of immunoregulatory cytokines (IL-6, IL-10, TGF-β) and the upregulation of M2-type molecules.
Moreover, in vitro macrophage polarization did not account for SARS-CoV-2 permissivity, since infection in M1- and M2-type macrophages were similar. In the study group of 76 COVID-19 patients with mild to severe clinical expression, all circulating monocyte subsets were decreased, probably due to massive emigration into tissues. Monocytes from these patients showed decreased HLA-DR expression and increased CD163 expression, regardless of clinical status.
SARS-CoV-2 infection induces immunoparalysis of the host
SARS-CoV-2 infection triggers the production of immunoregulatory cytokines, IL-6 and IL-10, in monocytes and macrophages and elicits a transcriptional program in macrophages enriched with M2-type genes. The numbers of classical, intermediate, and non-classical monocytes decreased in COVID-19 patients, regardless of the severity level. They also expressed significantly higher levels of CD163, a molecule associated with immunoregulatory phenotype than that expressed by healthy controls. However, HLA-DR was decreased in these patients.
The results of the study led to the conclusion that SARS-CoV-2 infects circulating monocytes and macrophages and induces immunoparalysis of the host, thus aiding COVID-19 disease progression. This is reminiscent of previous study results on SARS-CoV-1 that showed how the virus infects human macrophages but does not replicate within the cells. Moreover, SARS-CoV-1-infected macrophages were detected in the lungs of SARS patients.
Studies show that post-mortem examination of lymph nodes and spleen of COVID-19 patients showed the presence of SARS-CoV-2 nucleocapsid protein in macrophages that express CD169. Although it was clear that monocytes and macrophages produce molecules such as ACE2, TMPRSS2, and ADAM17 that help recognize and internalize SARS-CoV-2, the ability of the virus to replicate inside these cells was still unclear. According to the research team, the results of their study supports the hypothesis of an abortive infection that is similar to SARS-CoV-1 but distinct from MERS-CoV replication in macrophages.
“In monocytes, SARS-CoV-2 elicited a transient program dominated by the upregulation of IFNα gene, while macrophages exhibited a more diversified transcriptional program associating inflammatory and anti-inflammatory genes, which shifted to an anti-inflammatory program of M2 type.”
Thus, SARS-CoV-2 infects monocytes and macrophages and induces a more sustained activation in macrophages without cytopathic effect. The results also show that the response of monocytes and macrophages to the SARS-CoV-2 virus is more complicated than previously thought, and by analyzing circulating monocytes, the team found massive migration to tissues and the remaining blood monocytes in repair mode. This observation may throw some light on the post-COVID-19 complication risk for conditions such as fibrosis.
*Important Notice
bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- Monocytes and macrophages, targets of SARS-CoV-2: the clue for Covid-19 immunoparalysis Asma Boumaza, Laetitia Gay, Soraya Mezouar, Aïssatou Bailo Diallo, Moise Michel, Benoit Desnues, Didier Raoult, Bernard La Scola, Philippe Halfon, Joana Vitte, Daniel Olive, Jean-Louis Mege bioRxiv 2020.09.17.300996; doi: https://doi.org/10.1101/2020.09.17.300996
https://news.google.com/__i/rss/rd/articles/CBMidmh0dHBzOi8vd3d3Lm5ld3MtbWVkaWNhbC5uZXQvbmV3cy8yMDIwMDkyMC9TQVJTLUNvVi0yLWluZmVjdHMtbW9ub2N5dGVzLWFuZC1tYWNyb3BoYWdlcy13aXRob3V0LWN5dG9wYXRoaWMtZWZmZWN0LmFzcHjSAXpodHRwczovL3d3dy5uZXdzLW1lZGljYWwubmV0L2FtcC9uZXdzLzIwMjAwOTIwL1NBUlMtQ29WLTItaW5mZWN0cy1tb25vY3l0ZXMtYW5kLW1hY3JvcGhhZ2VzLXdpdGhvdXQtY3l0b3BhdGhpYy1lZmZlY3QuYXNweA?oc=5
2020-09-21 02:45:00Z
CAIiEDYCSEp5Hs3bGHL7s9yJpSgqMwgEKioIACIQZdRflS9INK7zM5FkBi3R3CoUCAoiEGXUX5UvSDSu8zORZAYt0dww1bjIBg
Bagikan Berita Ini














0 Response to "SARS-CoV-2 infects monocytes and macrophages, without cytopathic effect - News-Medical.Net"
Post a Comment